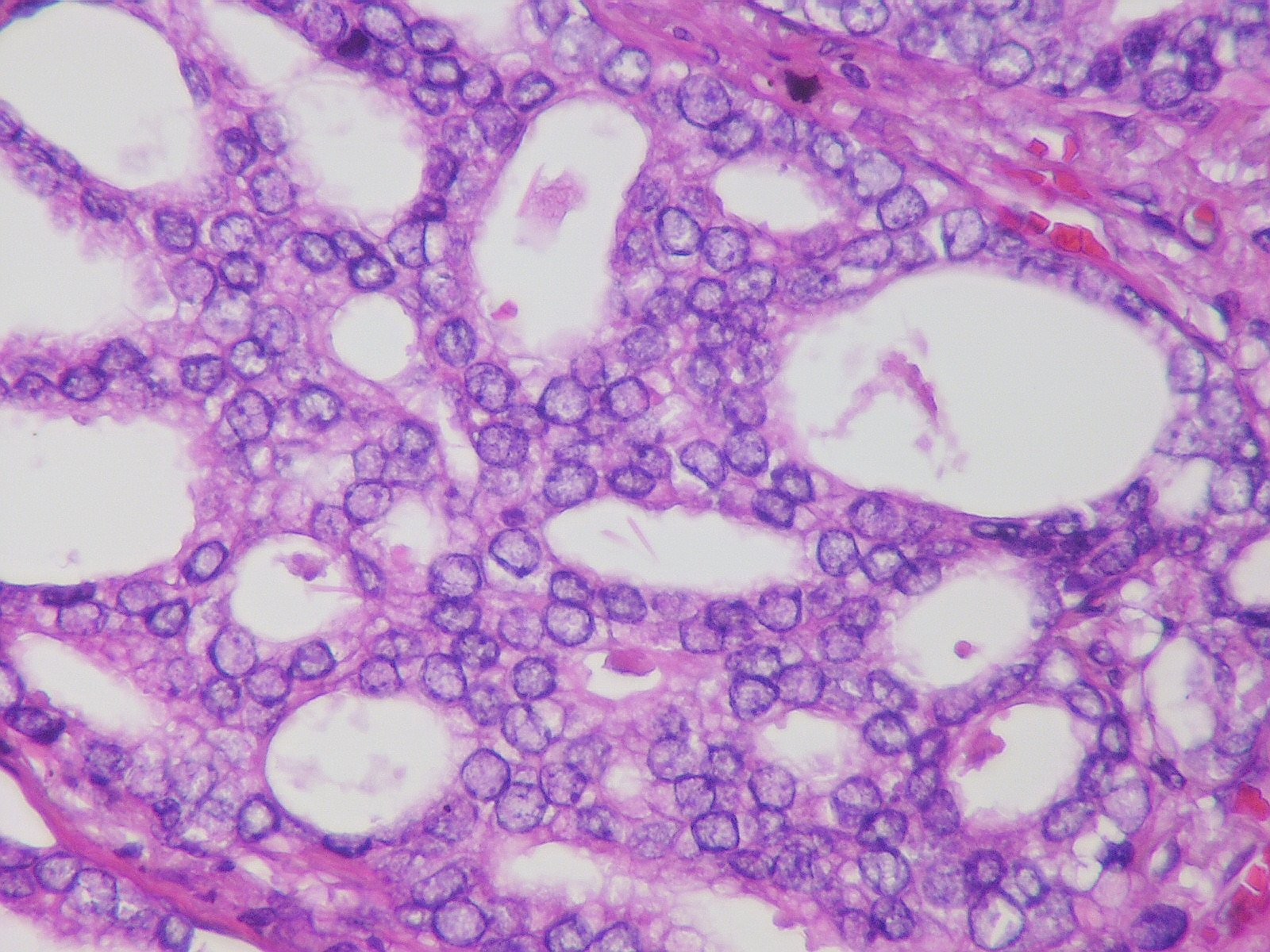
Prostate Cancer
Advertisement
Phase 3 EMBARK trial results show promising survival benefits of combination therapy in patients with nmHSPC.
Dr. Miles highlights the dilemma of managing PC with BCR after prostatectomy but no visible disease on standard imaging.
The panel discusses the evolving treatment landscape for mCRPC, focusing on the role of RLTs and PARP inhibitors.
The panel discusses treatment intensification strategies for mCSPC, emphasizing the shift toward doublet and triplet therapy.
The panel discusses the growing role of advanced molecular imaging and genomic testing in prostate cancer care.
The panel begins with the landscape of advanced PC care, emphasizing accurate categorization of disease stage and burden.
Drs. Ahmed and Ciuro discuss the real-world quality-of-life improvements patients may experience after de-escalating therapy.
Drs. Ahmed and Ciuro explore the rationale and complexity behind treatment de-escalation in de novo oligometastatic HSPC.
Dr. Kimura shares insights on PSMA PET/CT metrics and their potential role in guiding treatment decisions for mCRPC.
Dr. Flavell discusses how landmark studies have shaped current trial designs, and growing interest in combination therapies.
Dr. Flavell highlights the expansion of lutetium PSMA therapies into earlier disease settings: HSPC and oligometastatic PC.
Preliminary trial data suggests that 18F-DCFPyL PSMA PET/CT combined with mpMRI may improve csPCA detection over MRI alone.
Quantitative analysis outperformed visual analysis in the prediction of outcomes for patients undergoing Lu-PSMA therapy.
New research explores the prognostic potential of LuPSMA-SPECT/CT with RECIP 1.0 in patients with advanced prostate cancer.
Researchers investigated the use of PSMA PET-CT to determine treatment options for patients with biochemical failure in PC.
18F-fluciclovine PET detected disease in more than half of the study’s patients with BCR and negative PSMA PET results.
Drs. Swami and Ciuro discuss efforts to reduce disparities in the real-world application of mHSPC intensified therapy.
The panel concludes with a candid discussion about the most urgent changes that could improve cancer care today.
The panel explores the importance of introducing palliative care discussions earlier in the treatment journey.
The panel explores the overlooked burden of financial toxicity and proactively raising the topic with patients.

















 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.