ESMO 2023
Stay up-to-date with the latest coverage of the ESMO 2023 Congress.
Drs. Wallis and Sartor highlight issues with the control arm and considerations for treatment sequencing related to PSMAfore.
Dr. Sartor explains in detail the design and methodology, specific findings, nuances, and implications of the PSMAfore trial.
Dr. Nizam provides an overview of her recent research on biomarkers of TRAEs associated with EV for aUC.
Dr. Evan Yu explains the momentum for belzutifan in RCC, as well as the takeaways to be gleaned from the LITESPARK-005 trial.
Dr. Evan Yu gives a background on the successes of Lu-177 PSMA-617 and how the PSMAfore data add to our understanding.
Enfortumab vedotin shows promising activity and tolerability in cisplatin-ineligible patients with MIBC.
Niraparib and AAP led to improvements in TSP and PROs, and the combination is supported as a new first-line standard of care.
Investigating pembrolizumab plus enzalutamide in patients with chemotherapy-naïve mCRPC with or without prior abiraterone.
Results of the phase 2b SunRISe-1 trial showed TAR-200 yields complete responses in BCG-unresponsive high-risk NMIBC.
Belzutifan plus cabozantinib demonstrated durable antitumor activity and a consistent safety profile in patients with ccRCC.
Adding an adaptive dose of LuPSMA to enzalutamide was found to provide anticancer activity in patients with poor-risk mCRPC.
Results of the PSMAfore trial showed a statistically significant benefit in rPFS after [177Lu]Lu-PSMA-617 therapy.
Belzutifan is associated with significant improvement in PFS and ORR for patients with advanced ccRCC after previous therapy.
Drs. McGregor, Sonpavde highlight the Double Antibody Drug conjugate trial, updates from THOR, and other studies.
Drs. McGregor, Sonpavde share how the data compare across CheckMate 901 and EV-302, as well as considerations for physicians.
Paclitaxel administered with tremelimumab provided encouraging antitumor activity with a manageable safety profile.
The TAR-210 delivery system provides a continuous release of erdafitinib to the bladder while limiting systemic toxicities.
The practice-changing results were presented at ESMO 2023.
Nivolumab plus gemcitabine-cisplatin demonstrated meaningful improvements in OS and PFS as first-line treatment of mUC.
An LBA shed light on the effects of erdafitinib in patients with high-risk NMIBC with select FGFR alterations after BCG.
The LITESPARK study supports the use of 120 mg administered once daily as the preferred belzutifan dose.
Toripalimab plus axitinib resulted in a significantly long PFS and a high ORR in patients with previously untreated aRCC.
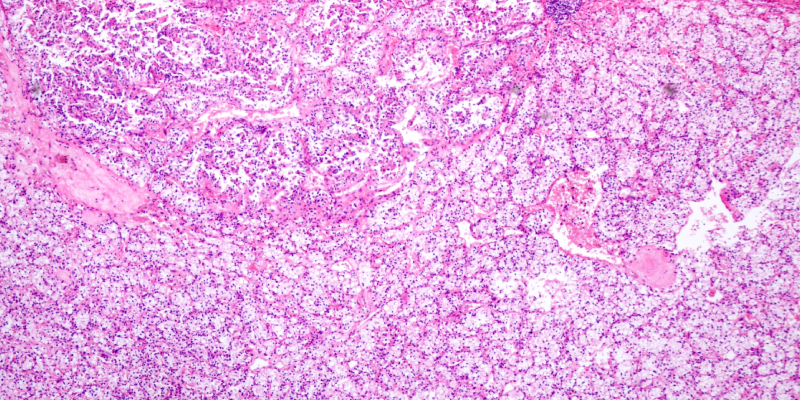
The current standard treatment administered after RP should consist of an observation policy with sRT for PSA failure.
The combination of sacituzumab govitecan plus enfortumab vedotin was found to be safe, and provides a high ORR.
PET patients were more likely to have a change in treatment compared with receiving CT alone.
Erdafitinib and pembrolizumab had similar OS rates, and toxicities were manageable with dose modifications.
A phase 2 study showed the safety of TKI discontinuation for patients with mRCC who respond to TKI/IO in the 1L setting.
Research shed light on the safety and efficacy of pembrolizumab plus enzalutamide and ADT in patients with NHA-naïve mHSPC.
ArteraAI Prostate—a multimodal, AI-derived prognostic test—was successfully validated in the phase 3 STAMPEDE trials.
New understandings of PROs related to enzalutamide-plus-leuprolide-acetate treatment for high-risk BCR nmHSPC.
A presentation on an individual patient data analysis of randomized controlled trials from the ICECaP consortium was given.
Xaluritamig was well tolerated with low-grade CRS and “encouraging” efficacy in heavily pretreated patients with mCRPC.
GU Oncology Now delivers the latest news in clinical trials, conference coverage, and more, highlighting advancements in genitourinary oncology treatments and tech.
Get conference updates straight to your inbox.












 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.